If today were the big day,
is your product ready?
We’ve seen it happen. A company develops a revolutionary medical device only to be denied during market approval. The roadblock? Non-compliance with industry standards.
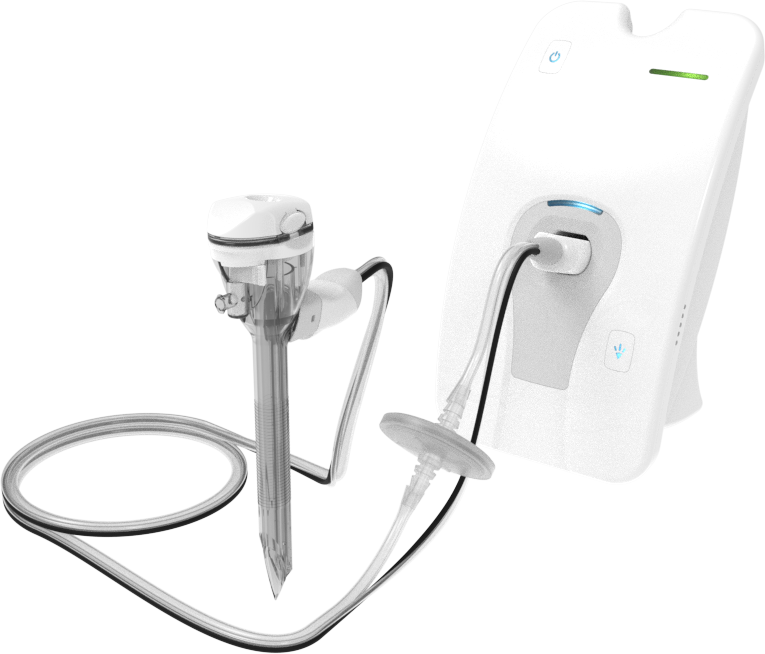
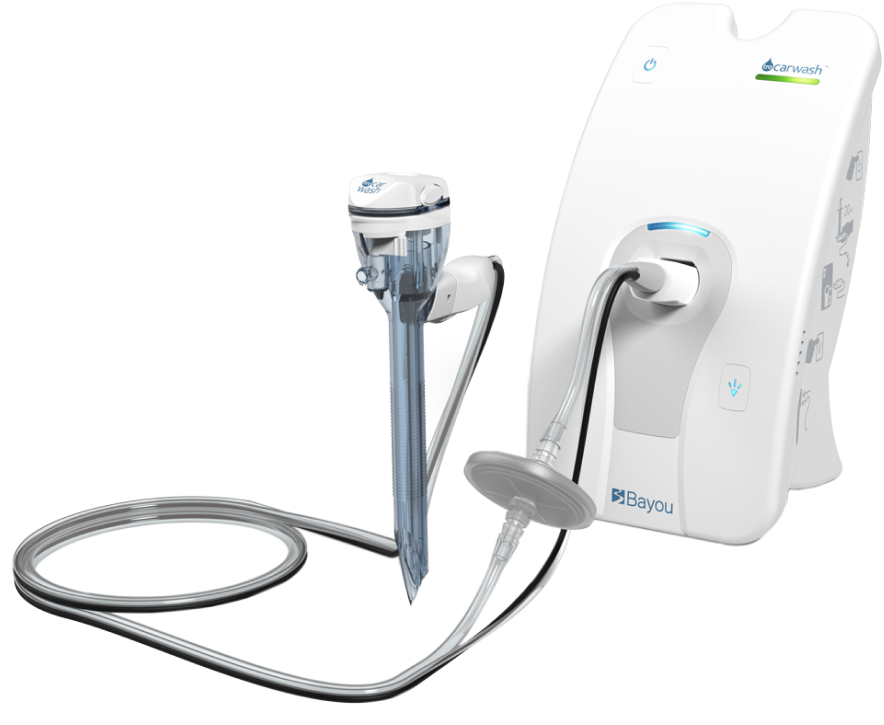


That's how our mission started.
Criterion aims to be the standard in product testing. We also strive to exceed our clients' regulatory needs.
As compliance and safety experts with years of experience testing and evaluating medical devices, we are committed to helping medical device manufacturers like you obtain market access for your product.
As the testing services arm of Biotex, Inc., a premier medical device development and manufacturing company with more than twenty years of success in the industry, Criterion has vast resources far beyond your typical test laboratory.
At Criterion, our goal is to save you time and resources and eliminate the costly redesign process. We'll review your product at any stage of development to ensure an understanding of your target market's regulatory requirements and provide the testing needed to demonstrate compliance.
Here’s how it works
01
We identify the specific regulatory requirements for your product.
02
We provide you with a detailed evaluation of your product for compliance.
03
We provide you with our recommendations to address your product's non-conformities in accordance with the requirements of the standard.
04
We'll ensure we are available to respond to your questions until your product passes regulatory requirements.
Explore our testing and consulting services
Testing Services
Criterion is your dedicated team of testing and regulatory experts to ensure your medical device passes rigorous compliance testing. Our goal is to help your device gain access to the market sooner.
Consulting Services
At Criterion, we can review your product at any stage of development and provide feedback to help minimize your risk of non-conformities.
Why We’re Different
We Think
Out of the Box
Working with companies of different sizes has helped us better understand the industry. As seasoned compliance and safety testing experts, we know not all circumstances come in pre-packaged boxes. At Criterion, we're always open to discussing your unique situation, and together, we'll develop the right solutions.
We Speak
Your Language
We have a unique perspective on compliance that none of our major competitors have. We're not only regulatory and safety experts. Being a part of Biotex, we also have experience designing, developing, and manufacturing medical devices. We can address your needs more effectively because we know the industry better.
We're Small
and Personal
Our small size works to your advantage as we conduct our business with a personal touch. We're accessible and ready to help without the unnecessary channels, paper trail, long queues, and wait time. We guarantee quality, accuracy, and expert recommendations to help your product enter the market sooner.
The Criterion Advantage
Saves you time and resources
Helps drive the design of your product
Minimizes the risk of non-conformities
Prevents the lengthy & costly redesign process
Talk to Us
If you have questions or need additional assistance, talk to us. Together, let’s come up with the best solutions.
Meet the Criterion Team

Wade Munsch
Director of Regulatory Affairs and Laboratory Manager
Wade does not only love cooking and working on cars in his downtime. He is also the Director of Regulatory Affairs for Biotex and the Laboratory Manager for Criterion.
Since starting his journey with Biotex in 2019 as the Regulatory Affairs Manager, Wade has become a significant force within the department. He continues to interface with clients directly and facilitates communication between development teams and regulatory agencies.
Wade is responsible for updating and maintaining quality system policies and procedures to ensure products comply with FDA QSR, ISO 13485, EU MDR, and other regulations. He also conducts internal audits to ensure everyone complies with regulatory standards.
Safety and quality have always been important to Wade, even when he had just started his career in Engineering. While attending college, he worked as a Tool Rental Associate at The Home Depot, helping customers learn how to use their tools and repairing their damaged equipment. After graduating from the University of Texas at San Antonio in 2009 with a degree in Electrical Engineering, he has held various roles in safety and compliance for different companies as a Product Safety Engineer.
When he's not busy helping clients with safety and regulatory concerns, Wade focuses his energy on home improvement. He enjoys remodeling the home he shares with his two kids and three dogs.
Being a foster parent is one of Wade's most important responsibilities, and he makes sure he nourishes his family with his awesome recipes and fabulous cooking. He also explores the outdoors in his spare time, testing his endurance by fishing, camping, hiking, and rock climbing.`

Nathan Slota
Compliance Engineer I
If you plan to go hiking with Nathan, you better gear up to endure a fifteen-mile trek. For the adventurous Compliance Engineer at Criterion, that's what an actual hike is all about. Anything less is not enough. When Nathan is not exploring the great outdoors camping, backpacking, and hiking, you'll find him working hard behind the scenes and on the frontline of Criterion.
Nathan is the primary author of Criterion's quality system, developed in compliance with ISO 17025 and the FDA ASCA program, proudly representing Criterion during its initial and follow-up accreditation audits since November 2021. He is the first mate to the laboratory manager, proactively ensuring Criterion’s day-to-day operations are in accordance with the quality system.
Bringing his experience and expertise as a Biomedical Engineer when he first started his journey with Biotex, Nathan performed various tasks as a Product Development Engineer before helping create Criterion and working his way up to the Compliance Engineer role for Criterion.
On top of testing and evaluating medical devices for compliance, Nathan handles client communications, sends quotes, and consults clients on developing appropriate design verification strategies. Nathan also supports Biotex’s ISO 13485 internal audits and verification and validation activities.
For Nathan, unwinding after a productive day at work means cooking a delicious dinner at home with his fiancé or testing new baking recipes. You may find one of their baked goods in Houston’s many charity baker collaboration fundraisers.
Remember Nathan's fifteen-mile real hike philosophy? He doesn't stop there. He also plays sand volleyball and bowling in a league. So, gear up. Expect a lot of energy when working with one of Criterion's go-getters.
Let's work together.
At Criterion, we are one with you in bringing a meaningful change to the world, so we can empower people, promote health, advance growth, and save lives. Let’s team up and work together to ensure your medical device passes compliance, enters the market, and becomes the catalyst for change the healthcare industry advocates.
The big day is coming
Let's get your medical device approved and ready for the market.